The Most Neglected NTD: Cryptosporidiosis
The 14-month-old boy arrived last January at University Teaching Hospitals—Children’s Hospital, University of Zambia, Lusaka, after suffering 12 days of diarrhea and poor appetite. He was lethargic and dehydrated, and had underlying severe acute malnutrition. Lab tests confirmed co-author Beatrice Amadi’s suspicion that the boy was infected with Cryptosporidium, a top cause of watery, profuse diarrhea in severely malnourished children.
Amadi, MD, prescribed nitazoxanide, currently the only available treatment for Cryptosporidium, though it isn’t particularly efficacious for children with severe acute malnutrition. She knew he had a high risk of dying. And even if he survived, he would be at high risk for stunted growth and poor neurological development.
Despite maximal care that followed WHO protocols, the child died after a week in the hospital.
Implicated in the heartbreaking toll of 200,000 child deaths annually, cryptosporidiosis receives far too little attention and should be added to the WHO list of neglected tropical diseases (NTDs).
Cryptosporidiosis is a significant public health issue that leads to acute diarrheal disease, malnutrition, developmental delays (including wasting and stunting), and death. The burden of the disease is disproportionately high in sub-Saharan Africa, South America, and Asia. The Global Enteric Multicenter Study (GEMS) leaves no room for doubt: Cryptosporidium is responsible for an astounding 7.5 million annual cryptosporidiosis cases in regions where mortality is highest among children under 5 years of age.
For any healthy adult, cryptosporidiosis presents as a bad case of watery diarrhea persisting for more than five days. Treatment with nitazoxanide, the only FDA-licensed therapeutic for persons older than 12 months, reduces those symptoms by a mere two days. But the drug’s efficacy is marginal at best in malnourished children. Just one in three children treated with nitazoxanide fared better than with a placebo, according to a 2001 Lancet article. Likewise, nitazoxanide shows no activity in immunocompromised hosts.
It's time for global action against this disease. As members of Cryptosporidiosis Therapeutics Advocacy Group (CTAG), we are calling for the following steps to be taken with great urgency.
First, new therapeutics are clearly needed for this deadly disease, given nitazoxanide’s lackluster record. Promising preclinical and clinical-stage candidates are in the pipeline. The leading one, developed by Novartis, is currently in Phase I clinical trials to assess safety. Six additional compounds, with various mechanisms-of-action, are being evaluated for efficacy in pre-clinical studies applying mouse and calf models.
Second, the WHO should add cryptosporidiosis its list of NTDs. This recognition would have multiple benefits. It would increase awareness of its impact on vulnerable populations and emphasize the need for quick action. Governments would be more likely to allocate resources and develop strategies for disease surveillance, diagnosis, and control measures, including prevention and control programs, and targeted interventions.
WHO’s recognition would also encourage collaboration between national and international agencies to collectively address the disease. It would attract the attention of researchers, academicians, and funders—crucial stakeholders in advancing scientific knowledge, developing innovative solutions, and securing funding for research and development activities. Pharmaceutical companies and vaccine developers would be more likely to invest in finding solutions. In addition, the disease’s place on the WHO’s NTD list would encourage collaboration between researchers, funders, and industry partners; and promote knowledge-sharing, resource pooling, and the exchange of expertise—all are essential for developing diagnostic tools, treatment options, and vaccines.
Finally, WHO’s recognition of cryptosporidiosis would encourage regulatory agencies like the U.S. FDA to prioritize evaluating and approving diagnostics, treatments, and potential vaccines. The FDA, for example, issues priority review vouchers (PRVs) to any company marketing an NTD therapeutic. A PRV restricts the FDA to a maximum review time of six months. This incredibly valuable voucher can be sold for more than $100 million or used by the company to beat a competitor to market on another product. It is a nice incentive to provide when developing a therapeutic targeted to a low-income country.
Achieving these crucial steps would give clinicians and public health practitioners the tools they need to reduce the devastating impact cryptosporidiosis on children worldwide.
The Cryptosporidium Therapeutics Advocacy Group (CTAG) is a global organization dedicated to finding new solutions to combat the disease and has been working to secure the disease’s addition to the NTD list.
CTAG authors of this commentary are:
Timothy A. Rapuano, MS, clinical trials project manager at the University of Washington Molecular Virology Laboratory; and president of the Organization of Regulatory and Clinical Associates (ORCA).
Nede A. Ovbiebo is a recent graduate of the University of Washington, with bachelor’s degrees in public health-global health and biochemistry.
Allison M.L. Crawford, M.Sc., research scientist at the University of Washington Molecular Virology Laboratory.
Beatrice Amadi, MD, MMed. Paed., PG Dip. Paed, gastroenterologist, co-director and a researcher at Tropical Gastroenterology & Nutrition Research Group;) and until recently, consultant pediatrician in charge of the Malnutrition Ward at the Children’s Hospital, University Teaching Hospitals, Ministry of Health; and honorary lecturer, School of Medicine, University of Zambia.
Wesley C. Van Voorhis, MD, PhD, CTAG leader; director of the Center for Emerging and Re-emerging Infectious Diseases (CERID); and professor at the University of Washington.
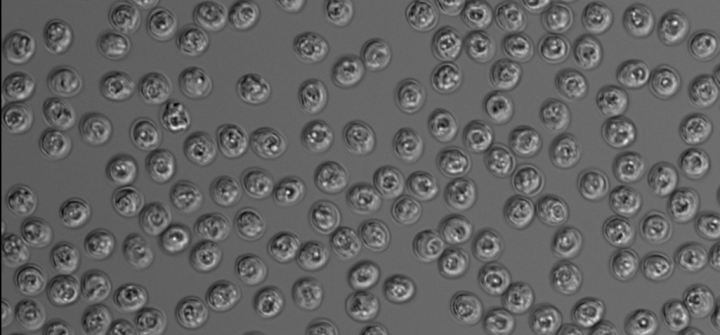
A light micrograph image of oocysts of the parasite Cryptosporidium parvum; each oocyst is roughly 5 microns in diameter. M. Kandasamy and B. Striepen