Why Do Prescription Drugs Have Such Crazy Names?
Wegovy. Zepbound. Prozac. Cialis. Xeljanz.
How do brand-name drugs get these names? And why do they sound so … bizarre?
There is an intricate method to the madness—with safety at its core.
If two brand-name drugs sound too similar, they can be easily confused or misused, with potentially lethal consequences.
“Look-alike, sound-alike” (LASA) medicines are recognized by the WHO as a leading global cause of medication errors, which in the U.S. alone cause at least one death every day and injure approximately 1.3 million people per year, according to the agency.
“The confusion can happen on the part of pharmacists, clinicians, or patients, so getting the names right is really important,” says Caleb Alexander, co-director of the Center for Drug Safety and Effectiveness at the Johns Hopkins Bloomberg School of Public Health.
In the U.S., where drug naming rules are among the world’s strictest, there are still drugs on the market with strikingly similar names that were “already out of the barn” when regulators began to tighten their drug naming rules, says Alexander. Consider the branded seizure medication Klonopin and clonidine, a generic blood pressure drug; or Lamisil—an antifungal used to treat yeast infections—and Lamictal, an anti-epileptic drug and mood stabilizer.
Today, the strictest drug-naming regulators—the U.S., the European Union, and Canada—have increasingly strict rules designed to help avoid confusion.
And brand consultants are frequently enlisted to come up with names that meet regulations, while creating a brand that’s “something you can latch onto,” says Scott Piergrossi, president of Creative at the Brand Institute. The firm is involved in naming three-quarters of FDA-approved drugs each year and the bulk of global drug names, too.
Naming a drug isn’t simple. It’s a years-long process that involves a cocktail of complex regulations, invented words, focus groups, tastes and trends, algorithms, and even AI.
How Drug Naming Works
Drugs have three types of names: chemical, nonproprietary (generic), and brand. Take 2-(4-isobutylphenyl) propionic acid. That is the chemical name for the generic drug ibuprofen, better known by its brand name, Advil.
Drugmakers establish a chemical name based on the rules of the International Union of Pure and Applied Chemistry. Generic names are designed to be used globally and are greenlit by the WHO’s International Nonproprietary Name program. They must include:
- A stem that indicates that what the drug does or its ingredients (E.g.: The profen in ibuprofen indicates it is an NSAID.)
- A prefix that distinguishes it from other drugs of the same type (ibuprofen as opposed to ketoprofen, another NSAID).
- No promotional language and explicit claims about what the drug does (which could cause confusion if the drug is used to treat multiple conditions).
- They must also avoid the letters Y, H, K, J, and W, which aren’t used in all languages that use the Roman alphabet.
But how does the generic semaglutide become Ozempic? Or duloxetine become Cymbalta?
Drug brand names are perhaps the trickiest naming challenge of all. While these names are not regulated by WHO, they must survive both regulatory and branding pressure tests, and be novel enough to trademark in all the countries where the drug will be sold.
But not all countries have strict or set guidelines for drug brand naming. Any of the top three global players U.S. FDA, the European Medicines Agency, and HealthCanada) can nix a name idea. Does it sound or look like another drug brand name? Is it overly promotional? Does it suggest anything offensive, inappropriate, or misleading in any of the markets where it will be sold? Those and other issues are all deal-breakers—but once a brand name has cleared by the Big Three, it will likely pass muster in other markets, perhaps with small changes to satisfy linguistic norms in that country.
But within these boundaries, there’s lots of room for creativity.
The Creative Process
Years before a drug hits the market, consultants are enlisted to come up with a brand name that not only satisfies regulators—but has a chance at becoming a household name.
Specifically, it should be easy to pronounce—ideally with five to nine letters, and two to four syllables.
The perfect name falls into a “white space”—a little corner of the drug lexicon that hasn’t been occupied yet, says Piergrossi.
One way to achieve that is to create a completely made-up word, known in the biz as an “empty vessel.” “That name doesn't necessarily mean anything, but you can still build the brand around it”—like Xeljanz, a drug that treats inflammatory bowel disease, says Piergrossi.
Or, it can be a little more on-the-nose, embedding words, positive associations, or subtle indications about what the drug does. “Something that you can latch onto from a meaning standpoint,” explains Piergrossi. That’s how the generic drug iptacopan—a factor B inhibitor used to treat a rare chronic blood disorder—came to be known as Fabhalta. The prefix ‘fab’ had two things going for it: It evoked both “fabulous” and “factor B.” The suffix “halta” suggests stop—a nod to what factor B inhibitors do.
As for explaining to the public how to say these brand-new, oddly spelled words, that comes later. “You have to teach pronunciation”—which is why commercials for branded drugs say the product’s name as many times as possible, says Piergrossi.
The ‘Big Bang’
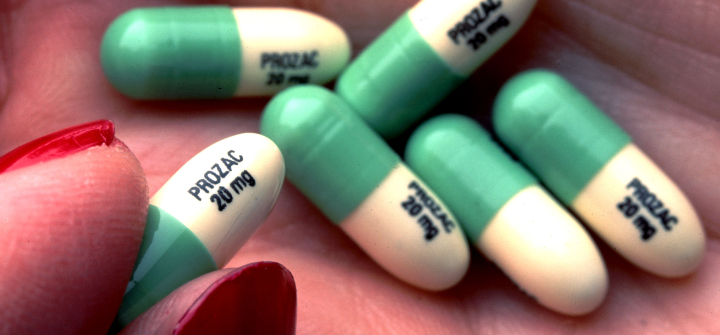
Branding drugs hasn’t always been so complex. But one drug changed everything: Prozac.
The iconic antidepressant, launched in 1988, marked a turning point in the industry, says Piergrossi.
It’s the ideal “empty vessel” name. It has no meaning related to antidepressants, and yet became synonymous with them. It went into the Oxford English Dictionary, and the title of a bestseller, Prozac Nation.
Before Prozac, getting a name cleared was more about trademarkability—whereas today, focus groups, consumer testing, and drug branding and advertising receive just as much attention as ensuring a name passes muster with regulators.
“Prozac was the Big Bang of the pharmaceutical naming universe,” he says. “It was powerful, approachable. It captured hearts and minds”—and it set the tone for the power of a short, easy-to-pronounce, invented name. The word itself was meaningless, but the ‘Pro’ prefix showed the power of encoding positive association into a made-up word. It showed the industry: “We’ve got to suggest. We’ve got to make it approachable,” says Piergrossi.
More Drugs, More Names
In the ever-expanding drugs market, finding a unique name is getting trickier.
The number of new drugs approved for sale in the U.S. between 2010 and 2019 rose by 60% compared with the previous decade, with a peak of 59 new drugs approved in 2018 alone.
When Piergrossi started in the drug-naming business in 2003, his team would come up with a couple hundred names per project. These days, they’re generating over 2,000 options, and only a handful will make it through to the “brutal” initial prescreen, which runs name options through an algorithm that tests it for similarities to existing drug names in all the countries where the drug will be marketed.
“It gets harder every year to pass” the algorithm test, he says.
In this increasingly complex market, AI has come at the perfect time, says Piergrossi. Gone are the days of creative teams gathering around a conference table with a stack of dictionaries and thesauruses, free-associating and speaking gibberish. Today, drug namers might instead ask ChatGPT, “What are novel ways to suggest inhibiting of something in the context of potentially a pharmaceutical agent?” Or “How else could you describe X? And you get some really actionable output,” Piergrossi says.
With thousands of names generated per project, the tech is a lifesaver rather than a threat to jobs in the drug naming business, says Piergrossi. “As soon as ChatGPT came out, I was hooked.”
Regulatory Gatekeepers
While the U.S., Europe, and Canada currently serve as the benchmark for drug regulations and drug naming, their naming conventions “are likely to … influence naming in in other countries,” says Alexander.
In India, where drug naming regulations are lax, activists have been calling on regulators to tighten drug naming rules to avoid harm to patients. For example, Indian physicians have expressed outrage that the drug Medzole is sold by four different companies to sell four different active ingredients, all for different medical conditions. “The medical community has been complaining about the problem for decades” but there is “zero political will” to fix the problem, wrote Dinesh Thakur and Prashant Reddy, authors of the book The Truth Pill: The Myth of Drug Regulation in India, in an op-ed published in The Hindu.
Brazil, however, is one country that has adopted stricter rules for naming drugs.
The country, which is—Latin America’s largest pharmaceutical market, “wanted to be in the global market,” and for emerging economies, tightening pharmaceutical standards, including names, to comply with the strictest regulators is a natural part of that evolution, says Domenica Redeschi, vice president of Brazil & Latin American Regulatory Affairs at the Drug Safety Institute, an arm of Brand Institute.
Through its drug regulator ANVISA, the country began implementing drug naming regulations in 2017, and enforcing them in 2019, broadly matching the rules of the FDA, EMA, and HealthCanada, and putting them in compliance with the WHO’s drug naming rules.
Prior to that, Brazil’s only drug naming rule was that it couldn’t have more than three letters in a row the same as another drug, says Redeschi.
In a globalized economy, the potential for confusion is greater if countries don’t share consistent naming conventions. “That’s a major safety issue,” she says.
But finding a name that agrees with multiple languages, and multiple regulators, poses another challenge, says Redeschi. In English-speaking markets, simply adding a silent “h” to a name can take a drug “from red to green”—but in Brazil, it’s another cause for confusion.
“In Portuguese, you pronounce every single syllable. There is no such thing as a silent letter,” Redeschi says. That’s why the popular drug for hot flashes, is known as Veozah in the U.S., and Veosa in Brazil.
Slang is a factor, too. One name floated for an antidepressant drug had the term “gozo” embedded into it. In some Spanish-speaking countries, “gozo” means to enjoy and have fun; in Brazil, it has a sexual connotation—which was problematic on two counts: It incorporated a crude slang phrase, and it would be easy to assume it was a drug for impotence.
“This obviously never made it to market,” says Redeschi. “There is the regulatory part which we need to follow. But there's also linguistic culture. What do you see when you look at this name? What comes to your mind? What's the connotation? It’s very important from a safety standpoint.”
More Than A Name
But Alexander points out that avoiding drug confusion is about much more than finding the perfect name. “We're all prone to errors. And we're all fallible,” he says. “That’s why it's so important to get these names right and to have systems that help support patients and clinicians and reduce the risks of inadvertent confusion across products.”
That means ensuring health workers and pharmacists are trained to use generic names alongside brand names, to know which products are prone to confusion (such as Klonopin and clonidine) and storing them separately from one another, and distinguishing different drugs not just by name, but by formulation and dose.
“It’s not as if you name it right, and then don’t need to think about it ever again,” he says.
Join the 50,000+ subscribers in 170+ countries who rely on Global Health NOW summaries and exclusive articles for the latest public health news. Sign up for our free weekday newsletter, and please share this link with friends and colleagues.
Tablets of the antidepressant Prozac. Paul S. Howell/Liaison Agency