The Superbug Fight Needs a Better Business Model
Antimicrobial resistance is already a leading cause of death worldwide—with a global burden of disease greater than HIV, malaria, and tuberculosis combined. Yet as AMR deaths spiral and the need grows for drugs that work against deadly infections, the pipeline for new antibiotics is drying up, not ramping up.
A recent study published in The Lancet found that 39 million people will die from superbugs worldwide between 2025 and 2050 unless action is taken, including developing more effective antibiotics and improving infection prevention and access to treatments.
By 2035, drug-resistant superbugs are expected to shorten global life expectancy by 1.8 years and cost $855 billion per year in health care costs and lost productivity, according to a report published last spring by the Global Leaders Group on Antimicrobial Resistance.
But the current pipeline for new antibiotics is “on the verge of collapse” within the next four to eight years—and a key reason is that antibiotic makers can’t stay in business, says Kevin Outterson, JD, co-director of Boston University’s health law program and executive director of CARB-X, a leading global nonprofit partnership focused on supporting the development of new antibacterial products.
Researchers say that the current business model for developing antibiotics—and getting them to where they’re needed most—isn’t fit for purpose. Countries are starting to get behind incentives that absorb some of the risk, and offer a significant return on investment. But funding for these programs still lags far behind what’s needed to save millions of lives, and billions of dollars in health care costs, and there are doubts about whether incentives led by wealthy nations will satisfy needs in low- and middle-income countries.
An Inhospitable Market
Worldwide, there are currently 20 clinical programs testing new antibiotics to target critical pathogens. Within the next two decades, Outterson predicts that number will dwindle to five. (For oncology, there are well over 1,000 such programs underway.)
Drug companies generally spend 10-15 years developing a new antibiotic. Many of these products fail along the way—“that’s just science,” says Outterson—but achieving regulatory approval and making it to market is no guarantee of financial success. Quite the opposite.
Governments and public health programs are highly conservative when it comes to doling out novel antibiotics, deploying them as little as possible to avoid the kind of overuse that leads to resistance. “That’s a perfect public health strategy, but a terrible business strategy,” says Outterson. “Imagine making a car, but then the government says, we want you to not sell that car for the first five years.”
Small pharmaceutical companies and startups now dominate the antibiotics space amid an exodus of Big Pharma companies—and this model has driven them into the ground. In the past two decades, seven publicly traded small antibiotic companies have received FDA approval for a new antibiotic. But “all of them have either gone bankrupt or been acquired in a distress sale in which the R&D investors lost everything. One hundred percent of them,” says Outterson.
These challenges have also gutted the pipeline of researchers. 82% of the researchers who were focused on innovative antibiotics in 2018, had left the field by 2023, according to a survey conducted by CARB-X.The total number of authors on all AMR publications has declined from a high of 3,599 in 1995 to 1,827 in 2020, according to a report published this year by AMR Industry Alliance. Today, there are some 3,000 active AMR researchers worldwide, compared to as many as 46,000 for cancer and 5,000 for HIV/AIDS, the report found.
To keep antibiotic makers afloat, researchers and their output need to be valued “not based on the volume of sales, but on their value to society,” says Outterson.
The Netflix Model for Antibiotics
Wealthy nations are starting to get behind mechanisms that absorb some of the risk of developing new antibiotics through “push” incentives that fund early stage antibiotic research and development, and “pull” incentives designed to keep the makers of novel antibiotics afloat regardless of how much of their drug is actually used.
So far the UK is the only country that’s signed on to a robust pull incentive program, says Outterson. Under a subscription-style service, two UK drugmakers will each receive $13 million per year for the next decade for unlimited access to their new antimicrobial therapeutics. Italy recently launched a similar program, and legislation for pull incentives has been proposed in the European Union. A bipartisan bill making its way through the U.S. Congress would invest $6 billion over 10 years in pull incentives. “That’s cheap compared to a future of no antibiotics,” says Outterson.
“Push” incentives—provided by organizations like CARB-X—support early-stage research and carry antibiotic candidates through their first human clinical trials, minimizing the risks companies face if their product doesn’t make it to market.
But push incentives worldwide are currently receiving only enough funding to produce two new drugs per decade, says Outterson. If governments invested more in push and pull incentives, more lives—and more health care costs—would be saved.
Outterson modeled two scenarios based on different levels of investment across G7 countries over 30 years:
- $150 million per year in push incentives + $1.5 billion a year in pull incentives = 16 new antibiotics, saving a million lives and a trillion dollars of economic value.
- $350 million per year in push incentives + $3 billion per year in pull incentives = 23 new antibiotics, saving 1.2 million lives and $1.3 trillion.
The cost of maintaining the status quo? “Pipeline collapse,” says Outterson.
More Than Money
Developing and deploying new antibiotics is not just about countries writing checks to drugmakers, but designing agreements that ensure drugs reach those who need them most, says Rohit Malpani, policy adviser at the Global Antibiotic Research & Development Partnership (GARDP), which works to accelerate the development of and access to treatments for drug-resistant bacterial infections.
Many of the antibiotics developed over the last few decades are not registered or available in most countries—and push incentives are primarily being designed and funded by G7 nations, says Malpani. “That makes sense because there are more resources there. But whether those countries are going to design incentives in ways that look beyond their own needs is a challenge.”
For its part, GARDP seeks to ensure that the fruits of antibiotic research reach the most vulnerable populations. For the past five years, GARDP has been focused on developing zoliflodacin, which, if successful, would be the first new antibiotic for treating gonorrhea in decades. GARDP has helped facilitate clinical trials for the drug in both wealthy countries and LMICs like South Africa and Thailand, and in high-risk populations including women with HIV and AIDS. GARDP’s partnership agreement with drugmaker Entassis Therapeutics also includes plans to obtain a license to commercialize the drug in up to 168 LMICs.
Malpani notes that it’s crucial that incentives be designed with this full public health picture in mind.
Protect the Antibiotics We Have
While improving the pipeline for new antibiotics is essential to fighting AMR, innovation isn’t everything, says Caline Mattar, MD, a leading researcher on AMR and infection prevention in resource-limited settings, and an associate professor of medicine at Washington University in St. Louis.
New antibiotics are needed to address bugs that are already entirely resistant to the arsenal of available antibiotics, “but as we develop new antibiotics, we need to find a way to protect them”—and that takes interventions across health systems to address the reality of how antibiotics are being used.
In theory, countries have agreed to do this. A UN High-Level Meeting on AMR in September updated countries’ commitments to tackling superbugs, setting a goal to reduce human deaths from AMR by 10% by 2030. But countries rarely honor these commitments. Under the previous UN resolution on AMR, in 2016, member states were mandated to develop National Action Plans to implement wide-ranging interventions against AMR—but 89% of member states have yet to fund or implement such plans.
On paper, over 90% of countries also have regulations to prohibit the sale of over-the-counter antibiotics. “But those regulations are often not applied,” says Mattar, particularly in LMICs, where there are often few treatments available to treat sick people and few qualified health care workers to correctly prescribe antibiotics. In these settings, antibiotics bought over-the-counter frequently become the default medication, contributing to resistance.
“When we have health care systems where the only available therapeutic for any ailment is an antibiotic, that is a health system where you’re never really going to be able to stop or slow down or reverse antimicrobial resistance,” warns Mattar.
To create a more sustainable future for antibiotics, ensuring that everyday health clinics and their staff have the resources and knowledge to minimize resistance, is just as important as developing new drugs, says Mattar.
Some key steps:
- Improve Diagnostics: In LMICs as well as high-income countries, delays in diagnosing bacterial infections contribute to the spread of AMR. If diagnostics are readily available at the point of care, health workers are better able to identify the right antibiotic, says Mattar. For example, rapid testing has revolutionized malaria diagnostics and enabled health workers to deploy the right medicines—and countries should invest in these tools to tackle drug-resistant superbugs, too, she says.
- Know What Works Where: It’s also essential to educate health care workers about which infections they are likely to encounter, “understanding exactly what type of resistant bacteria we are dealing with in every not just country, but rather region, district of a particular area, so that we know which antibiotics are going to work for infections there,” says Mattar. That might mean making better use of resources like the WHO AWaRe (Access, Watch, Reserve) antibiotic book, which provides guidance on the choice of antibiotic, dose, route of administration, and duration of treatment for more than 30 of the most common clinical infections.
- … And What Works Everywhere: One of the most important solutions to the AMR crisis is also the most basic: Making sure that health workers have access to soap, running water, and personal protective equipment. “When you have hospitals that don’t have electricity or running water, and health care workers cannot even wash their hands before going from one patient to the other—this is an issue that would not be solved with new antibiotics,” says Mattar.
Join the 50,000+ subscribers in 170+ countries who rely on Global Health NOW summaries and exclusive articles for the latest public health news. Sign up for our free weekday newsletter, and please share the link with friends and colleagues.
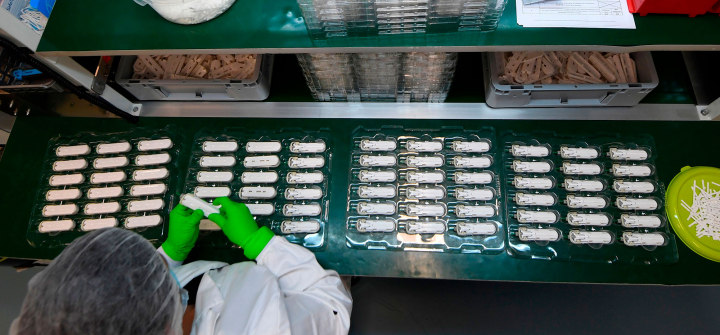
An NG Biotech employee manufactures "Carba" tests, an antibiotic resistance test in Guipry, western France. April 6, 2020. Damien Meyer / AFP via Getty