How Sickle Cell Disease and Malaria Defined Evolution
Sickle cell disease affects more than 20 million people worldwide and can be a devastating condition.
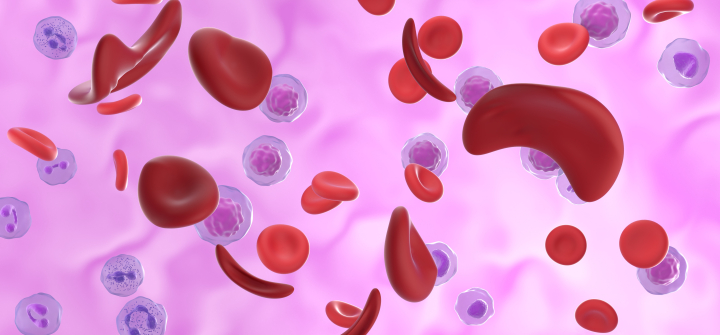
The inherited blood disorder affects the hemoglobin that carries oxygen through the body. It results in hard, sticky, banana or sickle-shaped cells that stick together, stifling the flow of oxygen. Left untreated, it can cause severe pain and potentially deadly health complications like infection, acute chest syndrome, and stroke.
But being a carrier of the sickle cell gene has had an evolutionary benefit: those with just one copy of the sickle cell gene avoid the worst symptoms of the disease, and are also protected against malaria.
The sickle cell gene evolved in Africa approximately 20,000 years ago, but there is still much to learn from the disease’s ancient genetic link to malaria.
Ambroise Wonkam, a Cameroonian physician, professor of medical genetics at the Johns Hopkins School of Medicine, and president of the African Society of Human Genetics, discusses how sickle cell disease and malaria marked human evolution in Africa and beyond, and how it highlights the importance of studying the African genome much more thoroughly.
Tell us more about sickle cell disease and its genetic connection between sickle cell disease and malaria.
The genetic link between sickle cell disease and malaria is a story of how our genome adapts to the environment.
Humans evolved in Africa 300,000 years ago. And at one point the Sahara desert was a big glacier. But when it melted, Central Africa became much warmer, creating an ideal habitat for mosquitoes. About 50,000 years ago, those mosquitoes, which initially infected primates, began to infect humans.
From time to time, humans have spontaneous mutations in our genes. And some 20,000 years ago, one of those mutations—the mutation for sickle cell disease—happened to be protective against malaria.
If you have one copy of that sickle cell mutation, hemoglobin-S, you are a carrier. You will not become sick from sickle cell disease, and you‘ll be very resistant to malaria. But if you have a double copy, one from each parent, you have sickle cell disease.
As Africa’s population evolved, those without the single mutation would often die of malaria, and those who had two copies of the gene would die of sickle cell disease. That’s why the single mutation became extremely common in Africa as populations settled, became more agriculturalist, and expanded.
What can the benefits of this specific single mutation teach us about malaria treatments?
We know the sickle cell mutation confers itself to malaria, but we don’t know exactly how. One theory is that when malaria infects red blood cells that have the sickle cell mutation, it doesn’t grow well as a parasite and will not reproduce itself easily. Another theory is that once hemoglobin-S—the protein that causes sickle cell disease—is infected with malaria, it is quickly eliminated from the blood and that malaria parasite will not grow. But we really don’t know. If we understood the specific mechanism of how the sickle cell mutation delays the progression of the malaria parasite in red blood cells, that would be a route for discovering new malaria treatments, because you can manipulate that.
Recent research has shown that malaria parasites may be trying to evade those protective genes from the sickle cell mutation. Tell us about that. Have the parasites been trying to do this for tens of thousands of years, and we are only now discovering it?
It’s possible they’ve been trying a whole time, and researchers just found it only recently.
Some parasites and bacteria have evolved over time along with our human genome in a process called co-evolution. For example, the first tuberculosis bacteria evolved somewhere in Ethiopia at the same time as humans. But migration impacted that lineage. The TB lineage that you see in Africa is not the exact same you see in Europe or in East Asia. If someone lives in Europe and gets infected by the East Asian lineage, they will be much sicker. So that means that there is some adaptation of those lineages to our human genome.
Now researchers hypothesize that the same co-evolution may have happened with malaria. It is possible that at some point, malaria also developed a mutation to be tolerant to humans. But we’re only just beginning to understand this. Those mutations that seem to evade the resistance to the sickle cell mutation were described very seriously only about two years ago, and that data was focused on The Gambia and Kenya.
It will be important to collect the same data from other areas where sickle cell mutation and malaria have coexisted for a very long time—like West Africa, India, or some parts of the Middle East—to see if there is the same pattern of changes.
Why does studying the African genome matter to everyone, regardless of whether they have the sickle cell mutation or are at risk of malaria?
Our human genome is like the library of life. There are three key elements that change its content: The direct environment, food, types of infection, and the mode of natural selection—of which sickle cell is just one example. Combining all of those factors, Africa has the most diverse genome of humankind—yet we know relatively little about it.
We are all African. That’s where we all evolved as humankind some 300,000 years ago. About 70,000 years ago, some of those people settled in the present-day Europe, Asia, and America, and so on. But the vast majority of that variation never moved out of Africa.
Yet currently, most studies on sickle cell disease are performed in Europe or America, where the populations’ genetic variation is only a fraction of what we’ll find in Africa.
What other factors make the African genome unique?
The African genome also matters because of Africa’s ecology. Europe and Asia are spread horizontally; but Africa sits on look the North- South axis, so it crosses many latitudes, more diverse climates, and types of food and infection.
For example, in the Mediterranean area people will drink milk. In the middle of Africa, they would not—so lactose resistance is very common in people in the middle of Africa, because milk was never really part of their diet. From migration, we see the same intolerance amongst African Americans.
Knowing our genome is also an equity imperative. If you don’t know the content of the variant that matters for disease, you can’t practice medicine equitably for all. That’s the scientific imperative of studying the African genome—we don’t have a choice as scientists and as a human society.
For a very long time people were not seeing it, but today, there is more awareness at the science and policy level, and you’ll see more investment in the next 10, 20 years into African genome sequencing.
Join the 50,000+ subscribers in 170+ countries who rely on Global Health NOW summaries and exclusive articles for the latest public health news. Sign up for our free weekday newsletter, and please share this link with friends and colleagues.
Illustration of red blood cells affected by sickle cell disease. Nemes Laszlo/Science Photo Library/Getty Images