This Widely Available Antibiotic Could Save Millions of Moms
A remarkable study published earlier this month offered up something we don’t see enough of in global health: really good news, with relatively few caveats.
Spanning 7 countries, a major piece of global maternal health research led by the University of Alabama found that a low-cost, widely available generic antibiotic can prevent life-threatening sepsis during childbirth in women delivering babies in low- and middle-income countries.
Sepsis—an extreme response to infection that triggers a chain reaction throughout the body—is a leading cause of maternal death. But this study’s authors found that a dose of azithromycin reduced the occurrence of maternal sepsis by ~35% and could help prevent the condition in as many as 2 million pregnant women each year. The medication also lowered the risk of maternal endometritis, infection, hospital readmission, and unscheduled medical visits.
Former CDC director Julie Gerberding, CEO of The Foundation for the National Institutes of Health, which managed the study, spoke to GHN about why this drug ticks so many boxes, and what needs to be done to get it deployed widely.
Beyond the medicine itself—which showed remarkable results in this study—what are the other advantages of this intervention?
It’s a cheap medicine. It’s generic. It’s available. It doesn’t require refrigeration. It’s also a single dose—rather than an injection. It’s also a general antibiotic that’s already used for other things, like preventing the eye disease trachoma.
The 3 main reasons that women die of childbirth are that they have an infection, that they hemorrhage, or that they have eclampsia—seizures associated with hypertension and kidney disorders.
The bleeding disorder is partially addressed by using medicines that stop bleeding, which are now available in a heat-stable format, so you don’t have to put them in the cold chain and they are easier to use in developing countries. A similar study was done with a heat-stable version of a blood-clotting drug—carbetocin—that is now on WHO’s essential medicines list. If that drug becomes available for broad use, it would help minimize a major cause of maternal mortality.
Similarly, this study really helps take sepsis off the table as another major cause of maternal mortality and the process would be similar to how other interventions for maternal mortality have been addressed.
How does that work? What happens next?
Now the WHO will look at these data; they’ll make recommendations, and I would expect that azithromycin would end up on the essential medicines list for vaginal delivery. Once that happens, most countries will make it available.
The uptake will take a while and I can’t say what the exact timeline would be for this process.
What was the standard of care for preventing maternal sepsis in LMICs before these findings?
There was really no consistent standard of care applied anywhere, because there is really no data to show what drug and what approach made the most sense.
Generally, even in LMICs, antibiotics are used around the time of a caesarean section procedure to prevent the complications of infection.
But the important point here is that the women in this study intended to deliver vaginally, and for those women it is not a normal standard of care to give antibiotics around the time of birth.
To show that a single dose of a very affordable and easily made available medicine can reduce sepsis in these women by 35%—that is a huge impact.
Are there concerns about drug resistance?
We never want to over-utilize antibiotics, which fuels antimicrobial resistance. When it is just a single dose, though, you know that resistance pressure is much less.
Another phase of the study will assess drug resistance among mothers and babies. I wouldn’t expect to see persistent drug resistant problems, but we have to look for it, and that will be important information.
Over time, the background of drug resistance in the community could increase, and that would have an impact on the long-term efficacy of the intervention— not necessarily because of this particular intervention for preventing sepsis, but because the medicine is used for other things.
That’s the problem with drug resistance. It doesn’t stay confined. It tends to move throughout the population, so long-term this could potentially render it less effective.
If that happens, there may be other broad spectrum, single-dose medicines that could be substituted for it in the future.
How can health systems be equipped to make the most of this drug’s potential?
This intervention doesn’t require an injection, making it much more feasible to think about widespread uptake—which is always the challenge in global health.
Even with something simple like a mosquito bed net, it’s often a long and complex process to really get the awareness, the support, the supply system, and the understanding of why and how to use the intervention.
I think the real solution here is to improve the overall experience and safety of birth practices in these areas. Sometimes women give birth at night at home, with no lights and no qualified attendant. We need to get more mothers into better systems of care with more hygienic, assisted delivery in health care settings instead of homes.
And for women who don’t have access to a health system, or resist going to a health center to give birth—could they potentially be given the given the antibiotic to take at home?
That’s a model that’s been used for other infections, including prevention of perinatal HIV transmission—so there may be a model for that.
Many communities now have home health aides, and this would be the kind of medicine that those workers could capably dispense.
In the release for this study you specifically thanked the women who participated in this study amid the COVID-19 pandemic—what was that process like?
The diversity of the populations included—spanning 7 countries (Bangladesh, the Democratic Republic of Congo, Guatemala, India, Kenya, Pakistan, and Zambia)—was a remarkable strength of this study. It meant that the results are more generalizable, and that’s really helpful for making decisions about how to approach this as a standard of care.
It’s hard enough to do health research in lower-income environments where there’s so many other competing priorities. But to do that during a pandemic, when the workforce is already preoccupied, or ill, or in quarantine—it’s a miracle that the investigators were able to enroll the patients and to follow them to assess their outcomes.
Big problems get solved by big villages, and I think that’s what happened here.
This interview has been edited for length and clarity.
Join the 50,000+ subscribers in over 170 countries who rely on Global Health NOW summaries and exclusive articles for the latest public health news. Sign up for our free weekday newsletter, and please share the link with friends and colleagues.
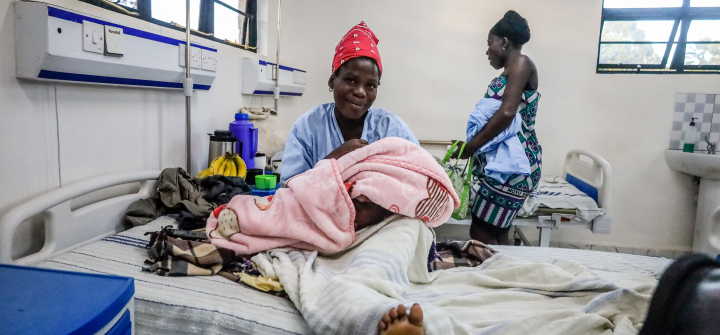
Young mothers care for their babies inside a maternity ward in Kibera, Nairobi, Kenya. November 7, 2022. Donwilson Odhiambo/Getty